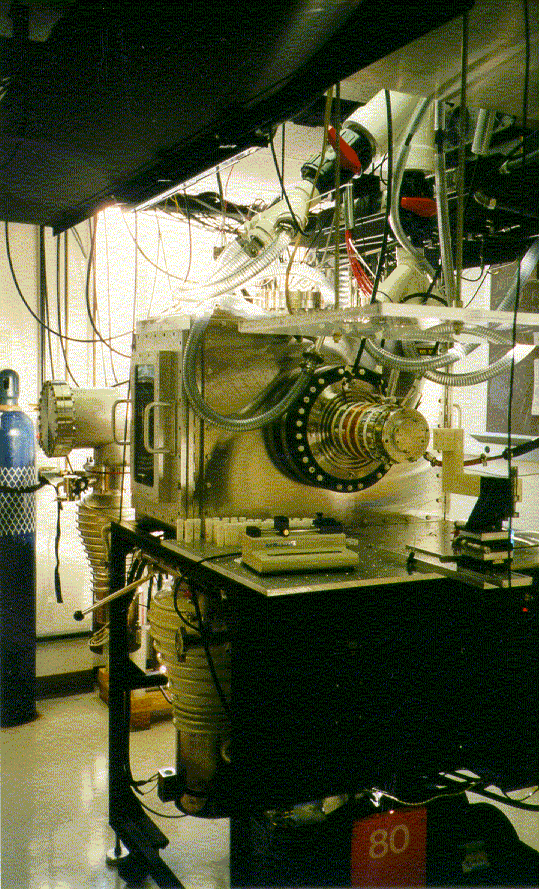
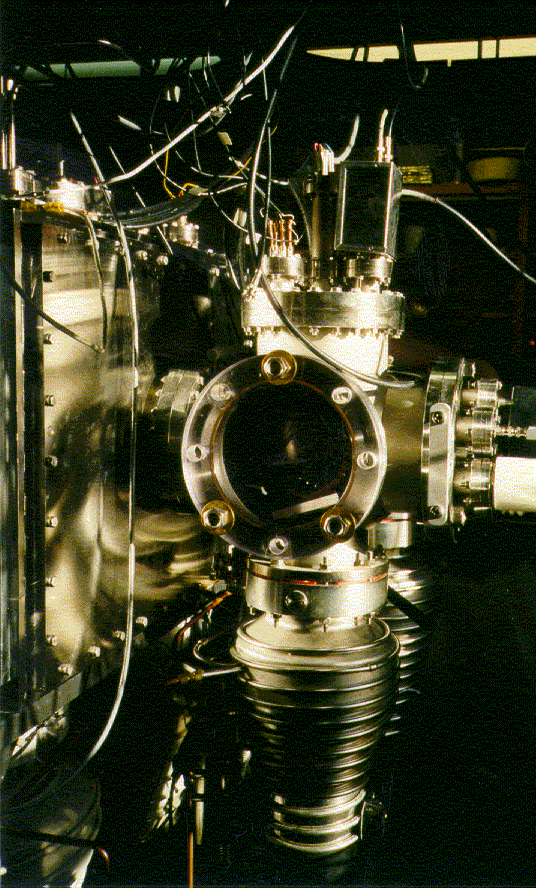
The Clemmer Group has, and continues, to devote a major effort toward the development of new instrumentation with two primary goals in mind:
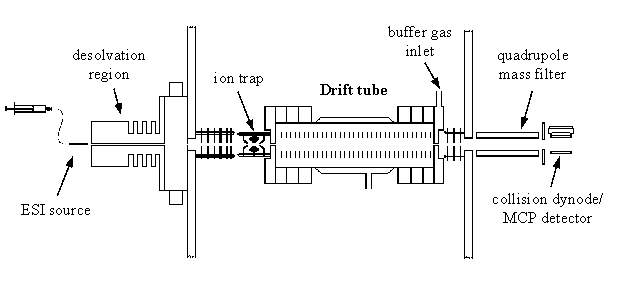
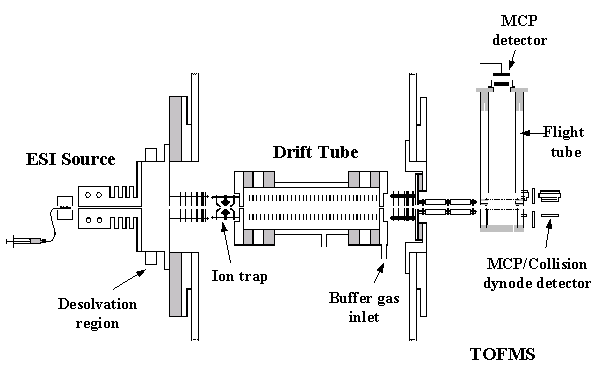
Our instruments are constructed in a modular fashion such that many components are interchangeable. Pictures and more information on some of our original custom designs can be found below.
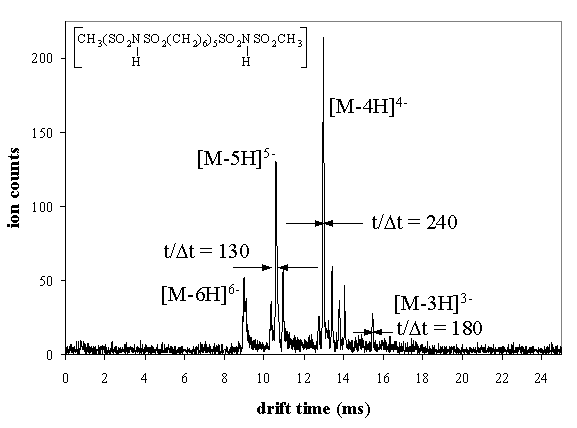
Biomolecular mixtures containing more than a few components become increasingly more difficult to analyze as the number of components increases. The presence of different charge states (in the case of electrosprayed ions), sequence isomers, and other species having overlapping mass spectral peaks makes complete assignment of components impossible by mass spectrometry alone.
The timescale of ion mobility (milliseconds) provides unique opportunities for multidimensional separations. Nesting ion mobility separation between condensed phase (seconds-to-minute timescales) separations and time-of-flight mass spectrometry (microsecond timescales) is particularly desirable because of the different modes of separation involved in these methods.
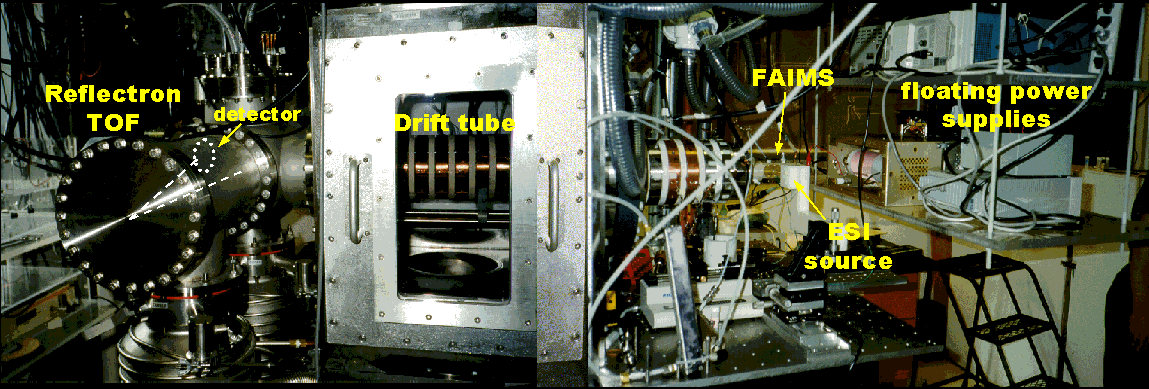
Current projects in this area include interfacing HPLC and CE separations with our existing ion mobility/TOF apparatus and investigating the capabilities of a FAIMS (field asymmetric waveform ion mobility spectrometry)/ion mobility/TOF arrangement (originally in collaboration with Guevremont and coworkers). A picture of the FAIMS/ion mobility/TOF instrument is shown below.
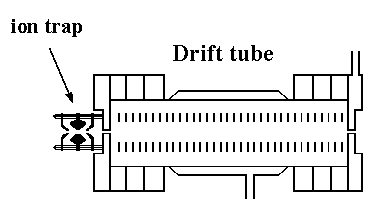
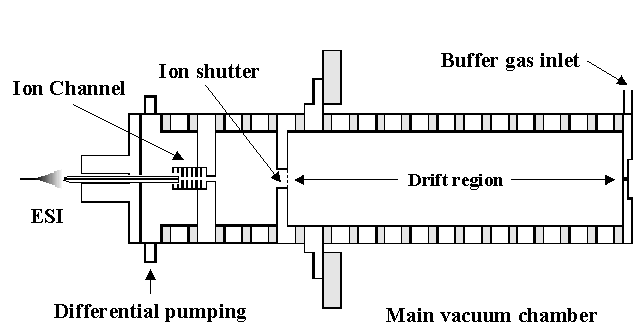

The main advantage of this design over the injected-ion drift tube is the increase in resolving power that is attained. The resolving power of the drift tube is based on the following expression, where we can calculate resolving power from the drift time (tD) of the peak, and the width of the peak in drift space (Δt). The remaining variables are the length of the drift region (L), the strength of the applied electric field (E), the charge on the ion (ze), Boltzmann's constant (kb), and temperature (T).
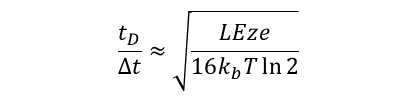
Thus, the resolving power can be increased by a factor equal to the square root of the increase in the electric field strength. At the low pressures (1–5 torr) used in the injected-ion drift tube, high electric field strengths cannot be achieved, due to the breakdown potential of the buffer gas. However, at higher buffer gas pressures, the field strength (and thus resolving power) can be increased. Another advantage of the high pressure drift tube is that ions are not injected; thus, the ions are analyzed in a much gentler manner, allowing conformations formed in the source to be preserved.