Nicholas A. Pierson
While IMS can be used purely as an added dimension of separation in analytical analyses, our group also uses IMS for fundamental studies of ion structure. Collision cross section (Ω), which represents the orientationally averaged shape of the ion, can be calculated from an ion�s drift time according to the following equation:1
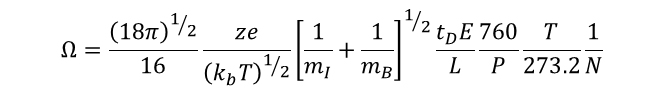
where kb is Boltzmann�s constant, T is temperature, mI is the mass of the ion, mB is the mass of the buffer gas (typically He or N2), tD is the ion drift time, E is the electric field, L is the length of the drift region, P is the pressure, and N is the number density of the gas at STP.
Structural characterization by IMS involves the study of different gas-phase conformations of biomolecular ions. The stability of these structures can be probed by varying trapping time, injection voltage, temperature, or by multidimensional IMS experiments.
Huilin Shi
Gas-phase protein ions retain — to some extent — their solution structure elements up to milliseconds after ESI. Structural analysis of gas-phase proteins may help determine the fundamental roles of intramolecular, sovent-molecule, and solvent-solvent interactions in establishing protein conformations. There are three primary protein states in the gas phase: folded structures, having cross sections that are similar to those that are calculated for coordinates of native structures from crystallographic and NMR data; partially-folded states, that are often observed as broad, unresolved features; and elongated conformers, having cross sections suggesting that little tertiary fold remains. Projects in this category include studies of: protein conformation, protein fragmentation, and protein unfolding when heated, collisionally activated, or upon time-dependent storage inside an ion trap.
Nicholas A. Pierson
IMS analysis of peptide conformation is complementary to solution-phase characterization techniques such as NMR, CD, and X-ray crystallization. In our laboratory, we typically analyze peptide ions produced by electrospray ionization (ESI).2 Projects in this category include studies of: peptide conformation, fundamental aggregation phenomena, peptide fragmentation, and gas-phase thermodynamics and kinetics.
Steven M. Zucker
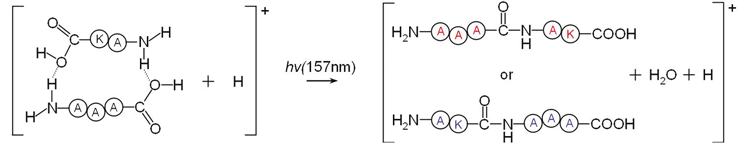
While investigating photodissociation of peptide ions on the IMS–hν–Q instrument, it was discovered that covalent bonds could be formed inside the mass spectrometer.3 Photoexcitation of proton-bound peptide complexes, such as the one depicted in the figure below, leads to the loss of water and the formation of a new peptide bond, possibly through the Norrish Type 1 reaction. The new peptide was fragmented with CID and produced the complete b and y ion sequence coverage of the peptide identical to that you would obtain from the commercially available peptide. This work was expanded beyond the coupling of peptides to include the synthesis of octapeptides from tetrapetides, intermolecular cross-linking of amino acid side chains, and glycosidic bonds formation between disaccharide complexes.4 Currently, work involves the formation of various peptides in a controlled manner to understand how this can yield insight into the origin of life.
Natalya Atlasevich
Chirality is a characteristic feature of life. Most biomolecules are composed of entirely L-amino acids or D-saccharides. Although chiral resolution of racemic solutions to form D- and L-crystals is a rare phenomenon, experimental evidence suggests that some small clusters of amino acids exhibit strong chiral preferences. Serine and proline clusters are two such systems. By varying the composition of the electrospray solution from enantiomerically pure (100% L or 100% D) to racemic (50:50 L:D), it is possible to delineate which cluster sizes prefer homochiral (resolved) or heterochiral (anti-resolved) compositions. Our research on the composition of proline clusters points towards the likelihood of chirally resolved sub-structures within larger racemic systems.5, 6, 7, 8, 9, 10
Full-text formats for many of these references can be found on the Publications page. External links are provided as necessary and available.