Feifei Zhu & Maissa M. Gaye
Glycomics refers to the comprehensive study of glycans (the carbohydrate moiety linked to a protein or to a lipid) in a given type of cell or organism. Glycosylation is the most frequent occurring posttranslational modification (~70% of human proteins are glycosylated). Unlike proteins and nucleic acids which form linear polymers of regularly repeating subunits, glycans are often branched structures, which can exist as multiple isomeric forms. Furthermore, the glycosidic bond is relatively flexible, thus multiple conformations of a structure are possible in solution. Our research in this area comprises: 1) the structural characterization of carbohydrates and glycans isomers 1, 2, 3 and 2) the development and application of methods for high-throughput comparative glycomics for disease state delineation.4, 5
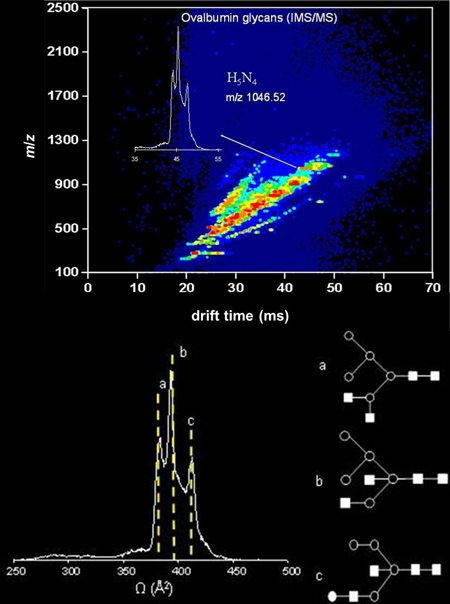
In an IMS�MS experiment, ions are separated based on their overall size and charge on a millisecond time-scale, thus complex mixtures of glycan isomers may be resolved in a high-throughput fashion. The nested IMS�MS distribution of N-linked glycans from chicken ovalbumin is shown above; the mobility distribution of the glycan ion H5N4 (m/z = 1046.52) is shown on a drift time scale as an inset, and on a cross section scale (Ω) underneath. The mobility distribution displays multiple features, which may be indicative of the existence of multiple gas-phase conformers or/and isomers. Three possible isomeric forms of this glycan ion are shown (a, b and c). The three features in the mobility distributions were assigned to each one of the three isomers on the basis of cross section calculation from molecular modeling simulations.
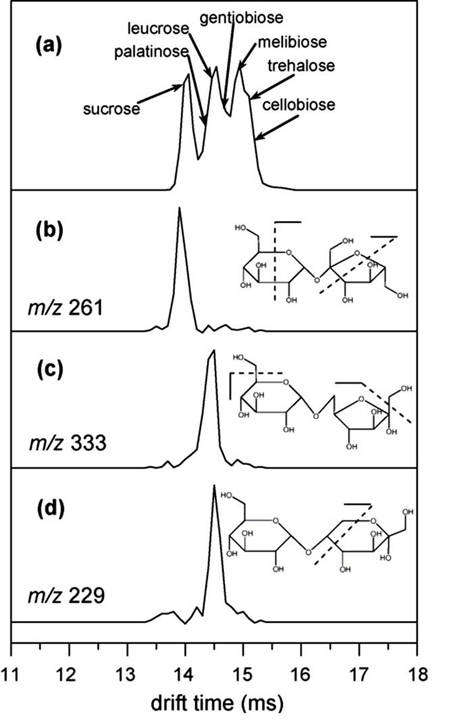
IMS combined with vacuum ultraviolet photodissociation (VUVPD) is used to identify individual components from a mixture of isobaric disaccharides. In this example, the seven isomers, which differ by the linkage position and/or the anomeric configuration between the two sugar units, are partially resolved by IMS alone (a). The VUVPD of a mobility selected ion produces specific fragment ion(s), allowing the characterization of the precursor ion from a complex mixture. The extracted fragment ion drift time distributions (b, c and d) are obtained from specific fragments for sucrose (b), palatinose (c) and leucrose (d).
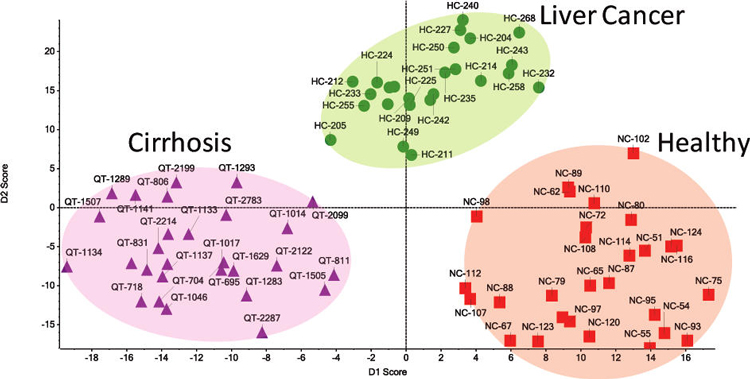
N-linked glycans are extracted from patient sera and analyzed by IMS�MS. We found that the mobility distribution of glycan ions provides a means of delineating disease phenotypes. In this example, patients diagnosed with liver cirrhosis (28 individuals), liver cancer (25 individuals) and a control group (28 individuals) are distinguished based on the combined mobility distribution of ten glycan ions.
Full-text formats for many of these references can be found on the Publications page. External links are provided as necessary and available.