Combinatorial libraries present a particularly challenging problem in mixture separation. The presence of sequence-, structural-, and stereoisomers at the same mass-to-charge (m/z) ratio prevents complete characterization of a complicated system by mass spectrometry alone. This problem is made tractable by ion mobility methods because of the differences in gas phase conformations that arise from the primary sequence or structure of isobaric components. Our laboratory has previously demonstrated the ability to resolve isomeric species from 676-component libraries of tripeptides using an ion mobility/time-of-flight (IMS–TOF) approach (see also the relevant Instrumentation section for more details on the experimental apparatus).
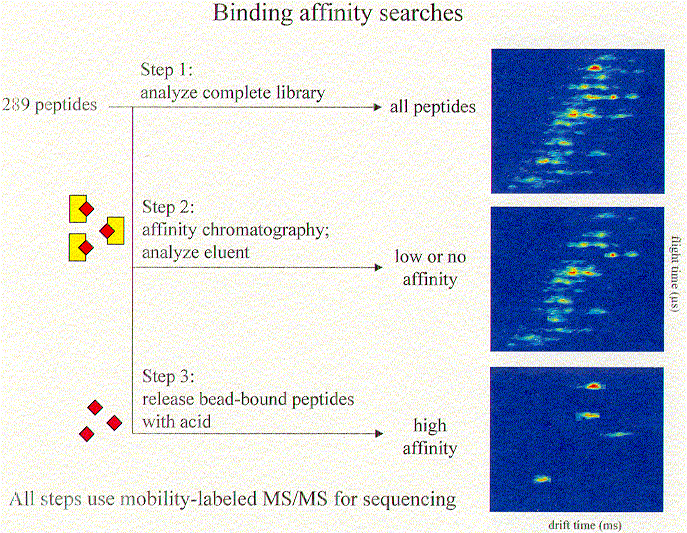
Once a combinatorial library has been analyzed for completeness, it can be used in binding affinity studies. We are currently using ion mobility/time-of-flight methods to investigate the binding affinities of synthetic library components for a specific target molecule. The general approach is diagrammed below, using a cartoon representation.